How Many Protons Are There in Any Te Atom
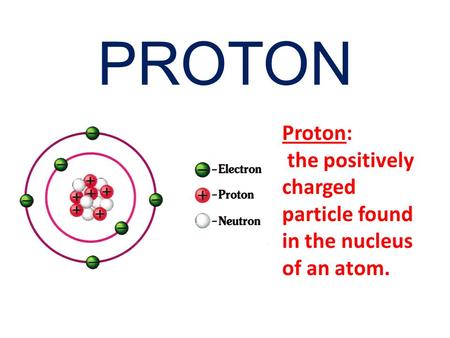
Protons are the positively charged particles in an atom while neutrons are subatomic particles that have no charge. Non-metal atoms gain electrons to form anions creating a negative charge.

The Atomic Structure In The Periodic Table
The atomic number Z determines total number of protons and uniquely identifies a chemical element also the atomic number is equal to the number of electrons.

. The number of protons is the same for all three 8 e List all of the things that are different about these atoms ignore the electrons. The closest noble gas to Te is Xe with 54 protons. Atom I 12 Carbon- 12 Argon-40 11 An atom with 13 protons and 13 neutrons Atom 2 13 12 Argon-41 Boron- 10 An atom with 14 protons and 13 neutrons Relationshi between atom 1 and atom 2 Isotopes C Same Atom Not Isotopes of Each Other Different Element CJ Isotopes e-Same Atom Not Isotopes of Each Other C Different Element Isotopes.
The atomic number of a chemical element represents the number of atoms contained in the nucleus of the atom. So from the given symbol we can tell that atomic number35. 9898 C 126295 K 181364 F Number of ProtonsElectrons.
An atom with 2 protons and 4 neutrons. X chemical symbol of the element. C How many protons are there in any Cl atom_____ d How many protons are there in any Te atom.
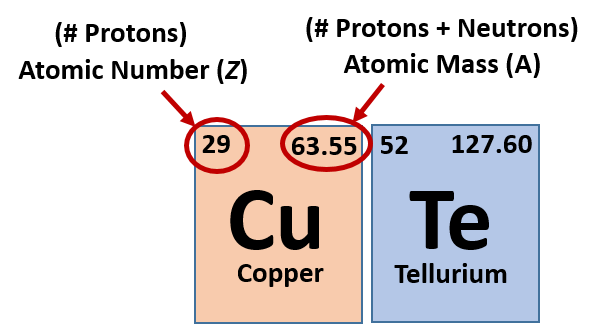
The atomic number Z. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. 1276 amu Melting Point.
Name Symbol Atomic number Mass Number Charge Number of neutrons 0 Number of Electrons 1 hydrogen-1 1 1 1 1H 2H 22Na sodium-22 10 24 12 12 12 25 13 47T1 108Ag 19F 6 6 carbon-13 carbon-12 carbon-13 carbon-14 7 5 4He 8 8 10 argon-41 18 18 70Ga 70Ga 5 10 2 5 6 5 3. Q-The mass of an electron is 91 10 31 kg. Tellurium is the element of the periodic table with atomic number 52.
_____ e Can you tell from the periodic table exactly how many neutrons are in an atom. How would you describe the obsession of zi dima. The chemical element chlorine has 17 electrons 17 protons and 18 neutrons.
Is 30 10 25 J calculate its wavelength. Play with the simulation to discover which. Hexagonal Density 293 K.
For our boron example 11 atomic mass 5 atomic number 6 neutrons. Tellurium is a chemical element with atomic number 52 which means there are 52 protons in its nucleus. 624 gcm 3 Color.
For zinc the number of protons is 30. To find the number of neutrons you will need to subtract the atomic number from the atomic mass. If you are given the atomic weight of an atom you need to subtract the number of neutrons to get the number of protons.
So as we know. The Te atom has 52 protons and 52 electrons. Popular Questions of Class 11 Chemistry.
C How many protons are there in any Cl atom_____ d How many protons are there in any Te atom. Complete the following table. Z Atomic numberNumber of protons.
Sometimes you can tell the elemental identity of a sample if all you have is the atomic weight. Check out a sample textbook solution. 17 d How many protons are there in any Te atom.
Therefore this means that an atom of. Complete the following table. Since we know that we have 35 protons and we also know the mass number ie- 79.
_____ e Can you tell from the periodic table exactly how many neutrons are in an atom. There are eighty-eight 88 protons in an atom. An atom with 4 protons and 4 neutrons.
The number of neutrons is different for all three atoms 16 17 18 the number of neutrons is different for all three atoms 16 17 18 14. How many protons are there in any Te atom. 4495 C 72265 K 8411 F Boiling Point.
Name Symbol Atomic number Mass Number Number of neutrons Number of Electrons Charge hydrogen-2 2H 1 2 1 1 0 3H. The element of an atom with 2 protons is always helium. 52 Number of Neutrons.
Q-Calculate the amount of carbon dioxide that could be produced when. Name Symbol Atomic number Mass Number Number of neutrons Number of Electrons Charge hydrogen-2 2H 1 2 1 1 0 3H. Remember that the atomic number is the same as the number of protons which you have already identified.
Why dont libraries smell like bookstores. Complete the following table. The total electrical charge of the nucleus is therefore Ze where e elementary charge equals to 1602 x 10-19 coulombs.
Isotopes Same Atom Not Isotopes of Each Other Different Element An atom with 13 protons and 13 neutrons An atom with 14 protons and 13 neutrons Isotopes Same. Assuming it is not an ion how many protons electrons and neutrons does an atom of Phosphorus haveQuestion 7 options15 protons 15 neutr. Up to 25 cash back Phosphorus has an atomic number of 15 and atomic mass of 30.

Protons Neutrons And Electrons Of A Carbon Atom Royalty Free Carbon Atom Model Atom Atom Model

Atom Song Atom Song Teaching Chemistry Teaching Science
An Atom Contains 11 Protons 11 Electrons And 12 Neutrons What Is Its Atomic Number And Its Mass Number Quora
Ch104 Chapter 2 Atoms And The Periodic Table Chemistry

Image Result For Carbon Atomic Theory Atom Diagram Proton Neutron Electron

Learn About The Model Of The Atom Electron Configuration Atom Model Aufbau Principle

Protons Neutrons Electrons Isotopes Average Mass Number Atomic Structure Atoms Vs Ions Youtube

Atomic Structure Protons Electrons Neutrons Youtube

See The Electron Configuration Diagrams For Atoms Of The Elements Atom Diagram Electron Configuration Electrons
Introduction To The Periodic Table Atomic Number Symbol Atomic Weight Element Compound Mixture Ppt Download

Atomic Structure Boundless Microbiology

What Is The World Made Of Ppt Download

Basic Chemistry Atoms And Ions Chemistry Basics Chemistry Education Teaching Chemistry

Protons Are In The Nucleus Center Of The Atom Have A Positive Charge Ppt Download



Comments
Post a Comment